Understanding Hot And Cold Corrosion In Marine Engines (Part I)
Wednesday, 30/03/2022, 08:29 GMT+7
The profit earned by the shipping companies does not solely depend on the cargo they are entitled to carry. In fact, a significant portion of the profit is achieved from the savings in operational costs, which enable the ship owners to provide competitive price for carrying cargo.
As everyone knows, one of the major operating costs for running a ship from Port X to Port Y is the fuel oil. And for the same reason, companies are doing everything possible to cut fuel costs to the minimum using techniques such as slow steaming with low-grade fuels.
However, the fuel oils that are used to run the marine engines also adversely affect the engine parts (due to the naturally occurring elements present in them) if not burnt efficiently.
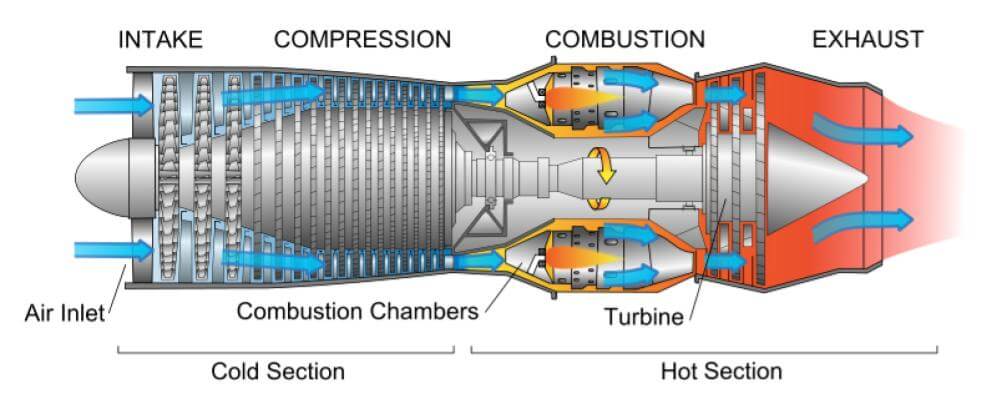
Corrosion is a primary concern on ships when it comes to burning heavy fuel oil in the marine engines. It can be further described as:
Hot Corrosion: It occurs due to the presence of Vanadium (Va) and Sodium (Na) in the fuel oil and affects exhaust passage of the engine.

- Vanadium is a naturally occurring element in marine fuel oils in soluble form, which means, it will not be separated even when the fuel is treated in the centrifuge. Vanadium, when combined with Sodium, can cause damage to the engine under elevated temperature. Sodium and Vanadium compounds are formed at a high temperature, which plays a crucial role in hot corrosion.
- The availability of abundant oxygen in the combustion chamber during the burning of fuel results in the oxidation of vanadium to form VO and VO2. During the temperature drop in the further combustion process, VO2 undergoes further oxidation resulting V2O5.
- V2O5 has a low melting point and becomes semi-liquid, sticky in nature and adhere to the surface they come into contact with.
- Sodium in the fuel reacts with water vapour during combustion to generate NaOH. This, in turn, combines with SO2 forming sodium sulphate.
- Sodium sulphate condenses at a temperature approx. below 890 deg. C and will adhere to surfaces with already present V2O5. This resultant deposits block gas passages and corrode metal surfaces. If the ratio of Va:Na is 3:1, the resulting complex melting point is at it’s lowest, which is about 350 – 450 deg C, and there is an increased likelihood of deposit formation.
Fuels with high vanadium and sodium will increase the tendency for deposit formation in the exhaust passages. At higher temperature (>600 deg C), ash deposits can accelerate corrosion of metals and fouling of gas passages.
Your Comments
Other news
Bản tóm tắt nhanh về kết quả kiểm chứng đầy ấn tượng của sản phẩm Mobil Delvac 1™ ESP 5W-40 trên động cơ tàu thủy hạng nặng Cummins KTA38 sau một thập ...
For profit, companies are doing everything possible to cut fuel costs to the minimum using techniques such as slow steaming with low-grade fuels. However, the fuel oils that are used to run the ...
For profit, companies are doing everything possible to cut fuel costs to the minimum using techniques such as slow steaming with low-grade fuels. However, the fuel oils that are used to run the ...
For profit, companies are doing everything possible to cut fuel costs to the minimum using techniques such as slow steaming with low-grade fuels. However, the fuel oils that are used to run the ...
Analysis on the dual fuel engines of the liquefied natural gas (LNG) tankers Spirit of Hela and Gigira Laitebo proved the viability of using a single lubricant, Mobilgard™ M430 engine oil, to ...







__gearbox_2_cde1648091.png)
__engine.jpg)
__Image_5-20-01.jpg)
